
What is Neon? Electron configuration, Atomic Number & Mass
What is Neon?
The Neon is a chemical element (gas) with an atomic symbol Ne and atomic number 10, it has a molar mass equal to 20.2 g / mol.
The word "Neon" comes from the Greek word "Neos" which means "new". This name would have been suggested to its discoverer, William Ramsay , by his thirteen year old son, who asked him for the name of this "new" gas.
In the periodic table, it is located in the second period , in the eighteenth column. Neon belongs to the family of noble gases. As it has 10 electrons then Its electronic structure is therefore as follows: (K)2 (L)8.
Below we are going to cover the properties & electronic configuration of Neon gas as well as its application.
Neon Properties |
|
|
Symbol of Neon |
Ne |
|
Neon Atomic number |
10 |
|
Family |
Noble Gas |
|
Group |
18 |
|
Period |
2 |
|
Block |
P block |
Atomic properties of Neon |
|
|
Neon Atomic molar mass |
20.2 g / mol |
|
Atomic radius |
0.16 nm |
|
Electronic configuration of Neon |
1s2 2s2 2p6 |
|
Electronic structure |
(K)2 (L)8 |
Physical properties of Neon |
|
|
Ordinary state |
Gas |
|
Neon melting point |
-249 ° C |
|
Boiling point |
-246 ° C |
Who discovered Neon?
In 1898 Neon was discovered by a pair of British chemists: William Ramsay and his assistant Morris Travers.
After his discovery of Argon, William Ramsay suspected the existence of elements of the same family, which are also chemically inert and therefore very difficult to detect.
The Linde-Hampson gas liquefaction process was developed in 1895. William Ramsay then used this process to liquefy air and subjected it to a distillation, which enabled him to discover not only Neon but also Krypton and Xenon.
Neon electron configuration
The electronic configuration of Neon is Ne: 1s2 2s2 2p6. The neon atom and C-4 , N-3 , O-2 , F-1 , Na+1 , Mg+2 , Al+3 , Si+4 , P+5 , S+6 have the same electronic configuration.
You can also write Neon electron configuration in abbreviated form i.e. [He] 2s2 2p6.
Neon electron configuration diagram
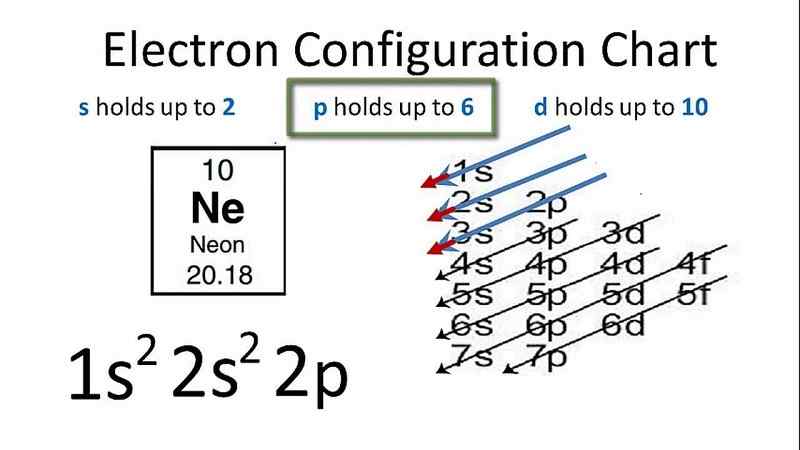
Electron structure of Neon-
Neon has 10 electrons, we can fill the electron shells as below:
-
2 electrons on the 1s-sublevel
-
2 electrons on the 2s-sublevel
-
6 electrons in the 2p sublevel
Isotopes of neon
19 isotopes of Neon are known today. Among the nineteen isotopes of Neon identified to date, three of them are stable and present in nature:
-
the isotope of mass number A = 20, which is the most abundant of the three, representing a little more than 90% of the abundance of Neon.
-
the isotope with mass number A = 21, largely in the minority compared to the other two (0.27%).
-
the isotope of mass number A = 22, representing about 9% of the abundance of Neon in nature.
Radioactive isotopes of Neon
The sixteen other isotopes of Neon are therefore radioactive isotopes: they are radioisotopes of Neon.
They have mass numbers between A = 16 and A = 34.
Among them, the isotope with mass number A = 24 is the one with the longest half-life, estimated at 3.38 minutes.
All the others have half-lives of less than a minute, or even less than a second for many of them.
Properties of Neon
-
Neon is the fourth most abundant element in the universe, only 0.0018% of the Earth's atmosphere is neon.
-
Neon is the second member of the rare gas family and, like all the elements of this family, it occurs in the form of a monatomic gas (= a single atom Ne) and is difficult to detect.
-
Neon only exists as a monatomic gas, it does not form a molecule and does not react. It is the basic property of chemical species belonging to the family of noble gases.
-
Its chemical inertia is linked to the particular stability conferred on it by its electronic configuration. This in fact makes any covalent bond unnecessary.
-
It is only present in very small quantities in the air, since its low density pushes it to rise and is lost in space.
-
Neon, like all other rare gases (Helium, Argon, Krypton, Xenon, Radon and Oganesson), has an electronic structure/configuration which gives it stability that does not allow it to form any kind of ions.
Important questions related to Neon
What is the oxidation state of Neon?
The oxidation state is the conditional charge of an atom in a compound. Neon atoms in compounds have an oxidation state of 0.
What are the quantum numbers of Neon?
We determine quantum numbers by the last electron in the configuration; for the Ne atom, these quantum numbers have the value N = 2, L = 1, M l = 1, M s = -1
What is the ionization energy of Neon?
The energy spent on the separation of an electron from an atom is called the ionization energy and is denoted by Eo . Ionization energy of Neon is Eo = 2081 kJ / mol.
What is the atomic mass of neon?
Neon has an atomic mass of 20.1797 g / mol. Its atomic number is 10 so it has 10 protons. Neon is a practically inert gas, so like all rare gases, it does not react with other elements.
What is the number of valence electrons in neon?
Neon has eight valence electrons like all rare gases. The last electronic layer of neon is full so it neither gives nor receives electrons.
What are the applications of Neon?
The major applications of Neon are related to the field of lighting.
- The major applications of Neon are related to the field of lighting.
- The reddish-orange color that neon emits in neon lights is widely used to advertise signs.
- Neon is widely used in different types of lamps such as signal lamps.
- Uses of neon include lightning interceptors, indicators, high voltage tubes, and television tubes.
Know about more periodic elements- Gadolinium, Germanium, Neon, Oxygen, Potassium, Promethium, Selenium, Sodium, Terbium, Tellurium, Yttrium, Ytterbium, Zirconium
Related Articales
Recently Posted
-
भगवान गौतम बुद्ध जीवन परिचय | Gautam Buddha in Hindi
December 15, 2022. -
कार्बन के अपररूप Allotropes of Carbon in Hindi
November 5, 2022. -
मिश्र धातु किसे कहते हैं? उपयोग, नाम, गुण Alloy in Hindi
July 27, 2022. -
गलनांक किसे कहते हैं? परिभाषा, उदाहरण Melting Point in Hindi
July 20, 2022. -
परिमाप किसे कहते हैं? Perimeter in Hindi
July 19, 2022.




