
What is Potassium? Electronic configuration, Atomic Number & Mass
Potassium, like its closest chemical element i.e. Sodium, have been known since antiquity and have been used in various fields of human activity. Potassium is a chemical element that is numbered 19 in the Periodic Table of the Elements.
Potassium Properties |
|
|
Symbol of Potassium |
K |
|
Potassium Atomic number |
19 |
|
Family |
Alkali metal |
|
Group |
1 |
|
Period |
4 |
|
Block |
s |
|
Volumic mass |
0.89 g.cm-3 |
|
Hardness |
0.4 |
|
Color |
Silvery white |
Atomic properties of Potassium |
|
|
Potassium Atomic mass |
39.0983 u |
|
Atomic radius |
220pm |
|
Electronic configuration of Potassium |
[Ar]4s1 |
|
Electrons by energy level |
2 | 8 | 8 | 1 |
|
Oxide |
Strong base |
Physical properties of Potassium |
|
|
Ordinary state |
Solid |
|
Potassium melting point |
63.5 ° C |
|
Boiling point |
759 ° C |
What is the symbol of potassium?
Although the spelling of potassium starts with P but the Potassium symbol is K, stange. There is a story behind this. The name of the element 19, potassium, derives from the neo latin Potassa which itself derives from the German Potaschen formed from the association of the terms "Pot" and "aschen" meaning respectively "Pot" and "ashes".
This is a reference to the method of preparing potash. Indeed, since antiquity, it has been obtained from plant ashes brought into contact with water in which soluble compounds dissolve and in particular potassium carbonate, the resulting solution is then filtered and then placed in a pot, so that the water evaporates and potash is formed.
It should be noted that at that time the term potash designates calcium carbonate while today it corresponds to a solution of potassium hydroxide. Regarding the symbol "K" it derives from the German name of this element "kalium".
The name potassium was proposed in 1807 by the discoverer of this element. So now question is-
Who discovered Potassium?
Some compounds, such as potash, have been known and used since a very old time but potassium was not identified as an element until 1807, when the British chemist Humphry Davy carried out the electrolysis of molten potassium hydroxide and obtained a still unknown metal.
Potassium was the first metal isolated by electrolysis, the decomposition of a chemical body under the effect of an electric current.
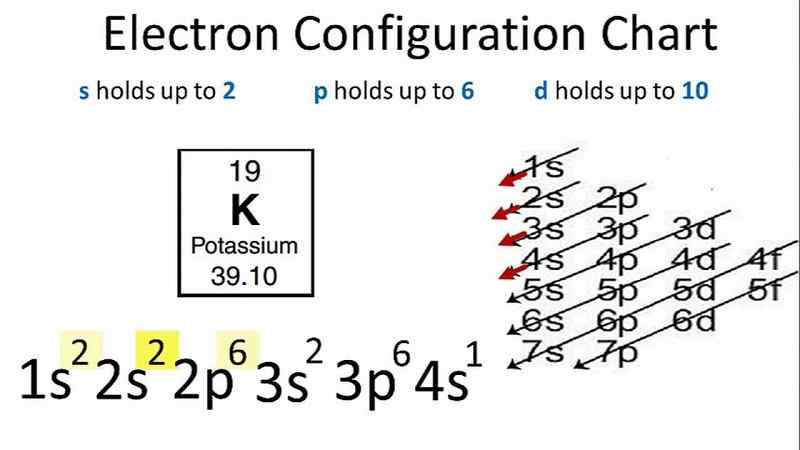
Electronic configuration of Potassium (K)
Potassium, with atomic weight 39.098 and atomic number 19, has the fundamental electronic configuration of 1s22s22p63s23p64s1. Also you can write potassium electron configuration in abbreviated form i.e. [Ar]4s1.
The potassium atom (K) and Cl-2 , Sc+2 , Ti+3 , V+4 , Mn+6 have the same electronic configuration.
Potassium has total 19 electrons, we fill the electron shells as below:
- 2 electrons on the 1s-sublevel
- 2 electrons on the 2s-sublevel
- 6 electrons in the 2p sublevel
- 2 electrons on the 3s-sublevel
- 6 electrons in the 3p sublevel
- 1 electron on the 4s-sublevel
- Electronic configuration of potassium Ion K+ : 1s22s22p63s23p64s0
- Potassium Ion K- electron configuration: 1s22s22p63s23p64s2
Diagram of Potassium electron configuration
Presence of Potassium
Potassium is a very abundant element in the earth's crust. Indeed, its Clarke is high, it represents 2.58% of the total weight of the earth's crust, making it one of the 7 most abundant elements on Earth.
However, potassium is not a native element since it is obtained, at least mainly, via the electrolysis of potassium hydroxide in a molten dry process. It is moreover this process which was discovered and used by Sir Davy.
There are many minerals composed of potassium such as:
- Carnallite- KMgCl3 6H2O
- Langbeinite- K2 Mg2 (SO4) 3
- Polyhalite- K2 Ca2 Mg (SO4 ) 4 2H2O
- And sylvine- KCl
Physical and chemical properties of Potassium
Potassium as a single body
It is a greyish metal, very soft, ductile and above all very reactive since it is one of the most reducing metals. It comes in a metallic form which can be obtained by electrolysis but which does not exist in nature.
Potassium is a very light, silvery white alkali metal, and it is very reactive with water.
For plants, the potassium ion plays an important role in maintaining osmotic pressure and in certain metabolic processes such as plant growth.
Potassium ions
Potassium ions, with the formula K+ , are positive monatomic ions, also called cations, carrying a positive excess charge.
It is the most common form in which potassium occurs in nature.
Potassium-based compounds
All potassium compounds are ionic in nature:
-
Potassium hydroxide (KOH) is an ionic compound formed from the combination of potassium ions i.e. K+ , and hydroxide ions i.e. OH- . It dissolves in water forming a basic aqueous solution called "potash".
-
Potassium chloride (KCl) is made up of potassium ions and chloride ions. It can be extracted from seawater or obtained from plant ashes. Its taste is reminiscent of kitchen salt, i.e. sodium chloride, without having its effects on blood pressure, it is therefore possible to use it as a substitute for the latter as part of a salt-free diet.
Isotopes of Potassium
Potassium has 24 isotopes with a mass number between 32 and 55. In addition to these isotopes, potassium also has 4 nuclear isomers.
However, natural potassium is only represented by 3 of its isotopes:
-
Potassium 39 representing 93.26% of natural potassium
-
Potassium 41, representing 6.73% of natural potassium
-
And a radioisotope, potassium 40, which has an extremely long half-life, representing 0.01167% of natural potassium.
It is important to note that potassium 40 is able to decay, producing beta+ and beta- radiation.
Uses of Potassium
Below are some of the common uses of potassium:
- Potassium is used in many chemical reactions but also in an alloy of sodium and potassium in order to become thermal conductors.
- It is mainly found in the form of compounds like potash, or potassium hydroxide, still used in the manufacturing of detergents.
- In agriculture, potassium can be used in the form of NPK fertilizer.
- Potassium hydroxide, more commonly known as potash, is used in the manufacture of detergents while potassium chloride is used, in the form of an infusion, for various purposes such as killing or treating heart problems.
- In pyrotechnics, it allows the coloring of fireworks in purple.
Health effects of Potassium
- It is possible to find potassium in our daily food, in particular bananas. A certain quantity of potassium necessary for the development of our organism. However, poor kidney function can be caused by a buildup of potassium, which can cause heart beat problems.
- You must also be careful with inhaling potassium, which can irritate different parts of the body such as the eyes, nose, throat but also the lungs, causing sneezing and coughing.
- Overexposure to potassium can have serious consequences since it can cause an accumulation of fluid in the lungs or even very serious burns.
FAQs
What are the oxidation states of potassium?
The oxidation state is the conditional charge of an atom in a compound, potassium atoms in compounds have oxidation states of 1, -1.
What is the valency of potassium?
The number of chemical bonds by which a given atom is connected to other atoms is known as valency, potassium atoms in compounds exhibit valence I.
What is the Ionization energy of potassium?
Ionization energy of potassium i.e K is E o = 419 kJ / mol.
What are the four quantum numbers of potassium element i.e. K?
The quantum numbers are determined by the last electron in the configuration. The four quantum numbers of the valence electron of potassium are N = 4, L = 0, M l = 0, M s = ½.
Know about more periodic elements- Gadolinium, Germanium, Neon, Oxygen, Potassium, Promethium, Selenium, Sodium, Terbium, Tellurium, Yttrium, Ytterbium, Zirconium
Related Articales
Recently Posted
-
भगवान गौतम बुद्ध जीवन परिचय | Gautam Buddha in Hindi
December 15, 2022. -
कार्बन के अपररूप Allotropes of Carbon in Hindi
November 5, 2022. -
मिश्र धातु किसे कहते हैं? उपयोग, नाम, गुण Alloy in Hindi
July 27, 2022. -
गलनांक किसे कहते हैं? परिभाषा, उदाहरण Melting Point in Hindi
July 20, 2022. -
परिमाप किसे कहते हैं? Perimeter in Hindi
July 19, 2022.




