
What is Oxygen? Electron configuration, Atomic Number & Mass, Uses
In this article you will get to know all about Oxygen element, like symbol & electronic configuration of Oxygen. Also you will know about atomic number & mass, valency and isotops of Oxygen. So lets start with:
General information of Oxygen |
|
|
Oxygen Symbol |
O |
|
Atomic number of oxygen |
8 |
|
Family |
Non-metals |
|
Group (column) |
2 |
|
Period (row) |
16 |
|
Block (electronic underlayer) |
p |
Atomic properties of Oxygen |
|
|
Oxygen Atomic mass (main isotope) |
15.9994 u |
|
Electronic structure of Oxygen |
(K)2 (L)6 |
|
Electronic configuration of Oxygen |
1s2 2s2 2p4 |
Chemical properties of Oxygen |
|
|
electronegativity |
3.44 |
|
Atomic molar mass (g / mol) |
16 |
What is Oxygen?
Oxygen is Omnipresent, omnipotent and invisible element. According to religious people, only God can be omnipresent, omnipotent and at the same time invisible. In fact, all these three epithets can be easily found in chemical element with atomic number 8 i.e. oxygen.
Oxygen is the most abundant element on earth: in the atmosphere it is about 23%, in the composition of water + 89%, in the human body + 65%.
OXYGEN is a gaseous substance contained in the air in an amount of 21% by volume and possessing oxidizing properties. It is one of the mandatory components of a combustible medium in case of fire and the formation of explosive steam, gas and dust-air mixtures.
After hydrogen and helium, Oxygen is the third most abundant chemical element in the universe. On Earth, Oxygen is present combined with various elements, in the form of mineral oxides or within the chemical functions of organic compounds (like- alcohol, ketone, carboxylic acid ...).
It is present in the air as dioxygen, it is also present in water combined with hydrogen and biological compounds. It is vital and most important for most living organisms on earth.
Difference between oxygen and dioxygen
Be careful not to confuse oxygen and dioxygen. Indeed, in everyday language we speak of the oxygen we breathe, but chemically it is dioxygen (2 atoms of oxygen).
For example, oxygen is a constituent of water (H2O, a single oxygen atom), but dioxygen also exists dissolved in water (which allows the respiration of aquatic organisms).
Structure of Oxygen
Under normal conditions of temperature and pressure, oxygen is odorless, colorless and its molecular formula is O2.
The two oxygen atoms connect together in an electron configuration in a triplet state. This bond is arranged in two and simplifies double bond or combination descriptions of a bond with two three-electron and two-electron bonds.
Origin of Oxygen
The word oxygen is formed by combining the Greek terms "oxus" and "gennan" which means "acid former".
This name was proposed by the French chemist Antoine Lavoisier in reference to the theory of the time according to which oxygen is the element common to all acidic compounds.
This theory has since been invalidated (it is hydrogen not oxygen that is responsible for the acidity), but the name stuck.
Discovery of oxygen: a brief history
Presence of oxygen and dioxygen on earth has always been essential to life, but it was not formally identified as an element until the end of the 18th century.
-
Leonardo da Vinci (artist, engineer, scientist, Florentine inventor) said that air is a mixture of gases and that one of them allows both human respiration and combustion. He had clearly identified the role of oxygen.
-
Michal Sedziwoj, Polish alchemist and doctor discovered that by heating saltpetre (made up of potassium nitrate) we obtain a gas which allows breathing and which he called "elixir of life". The reaction can be described by the equation:
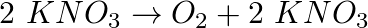
-
In 1773, Carl Scheele (Swedish chemist) obtained a gas by heating mercury oxide. This gas is capable of sustaining combustion and he called it "fire air".
-
The following year in 1774, Joseph Priestley, British theologian having carried out many experiments/works in chemistry and in physics, without being aware of the work of Scheele, carried out a similar experiment and named the gas which it obtains "dephlogisticated air". This term comes from phlogiston, the theory according to which fire finds its existence thanks to a substance called phlogiston, released during combustion. He published the results obtained in 1775, two year before Scheele (who published them in 1977), so he was officially credited with the discovery of dioxygen. However, without having really defined or identified it.
-
Shortly after, in 1775 Antoine Lavoisier, French chemist, one of the fathers of modern chemistry, shows that the gas discovered by Priestley represents a fifth of the total volume of air and that it allows the respiration of living beings. He proposed the name to it "oxygen". He also worked on the oxidation of metals, which allows oxygen to be identified as an element.
-
In 1883, dioxygen was liquefied for the first time by Polish chemists Zygmunt Wróblewski and Karol Olszewski.
Chemical origin of oxygen
Oxygen is an element of stellar origin. There are several routes of synthesis, which explains the presence of several natural isotopes of oxygen:
- Aged and massive stars have a helium nucleus, which at the end of its life heats up and allows the helium to merge with itself, as well as other elements present.
- In very heavy stars during the process of photodisintegration of Neon.
- Also in the stars, during the process of fusion of hydrogen into helium.
- More rarely, in evolved massive stars, when the star is sufficiently rich in nitrogen, this one is able to absorb a helium nucleus.
Read: Oxygen ki khoj kisne ki thi?
What is the symbol of oxygen?
In the earth's crust, oxygen is the most abundant element, constituting about half of its mass. Oxygen is the eighth element in the Periodic Table and is represented by the symbol O.
Oxygen Electron configuration
Oxygen in the periodic table takes 8th place, in the 2nd period. Electronic configuration of oxygen in detail is: O: 1s2 2s2 2p4 , also you can write oxygen electron configuration is abbreviated form i.e. [He] 2s2 2p4.
The oxygen atom and C-2 , N-1 have the same electronic configuration. Oxygen has 8 electrons, we can fill the electron shells in the order as below:
- 2 electrons on the 1s-sublevel
- 2 electrons on the 2s-sublevel
- 4 electrons on the 2p-sublevel
What group of the periodic table includes oxygen?
In VI group.
What is the oxidation state of oxygen?
Oxygen atoms in compounds have oxidation states 2, 1, 0, -1, -2.
What is the valency of oxygen?
The oxygen valence characterizes the ability of the O atom to form chemical bonds. Oxygen atoms in compounds exhibit valence II, I.
What are the quantum numbers of oxygen?
Quantum numbers are determined by the last electron in the configuration; for oxygen atoms, these quantum numbers have the value N = 2, L = 1, Ml = 2, Ms = ½ .
What is the Ionization energy of oxygen?
The Ionization energy of oxygen, E o = 1314 kJ / mol.
What process produces liquid oxygen?
liquefaction of air
By what process does free oxygen exist?
Photosynthesis
Isotopes of Oxygen
Natural non-radioactive isotopes
Oxygen has three non-radioactive natural isotopes ranging from mass numbers 16 to 18 i.e. 16O, 17O, and 18O. Isotope 16 is very largely present (99.762%), while isotope 17 is present at 0.038%. Thus the standard atomic mass assigned to oxygen is very close to 16 (15.9994 U).
These percentages of occurrence are averages on earth and may vary locally, due to planetary fluxes which more easily entrain light isotopes. In fact, isotope 18 will evaporate more easily and fall back more easily than isotope 16. This phenomenon is all the more true when the temperatures are cold.
Even if this difference remains in reality tiny, it is very widely exploitable for scientists because current equipment makes it possible to measure these differences quite easily. Thus, the water of the oceans close to the poles is poorer in O18 than the equatorial waters.
The study of the O18 / O16 isotopic ratio with respect to the mean can prove to be very useful for the understanding of certain phenomena.
Natural radioisotopes of oxygen
Oxygen does not have naturally occurring radioactive isotopes.
Synthetic isotopes of oxygen
Other isotopes of oxygen have been synthesized, ranging in mass numbers of 12 to 15 for the lightest and 19 to 28 for the heaviest. These isotopes are radioactive.
The lightest isotopes decay mainly according to a process of β+ radioactivity and the heaviest mainly according to a β- decay .
Oxygen ions
Oxygen does not exist as a stable monatomic ion in aqueous solution. On the other hand, in an ionic crystal, it forms the oxide ion of the formula O2- which has two more electrons than the atom.
Simple oxygen-based bodies
Due to the electronic configuration of its valence electrons, oxygen has six electrons available. Four electrons form two non-binding doublets. This leaves two electrons very available to react with other chemical elements, which is why oxygen can bind to most other chemical elements.
Therefore, we do not find the element oxygen in its native state on earth. However, we are surrounded by oxygen, which is why oxygen and dioxygen are often confused.
The main simple bodies formed from oxygen on earth are:
- Dioxygen (O2) is a molecule made up of two oxygen atoms. It is one of the two major gases constituting air (approximately 21%). It allows animal respiration, intervenes in combustion, causes the oxidation of most metals, but does not react with water or other constituents of the air.
- Ozone of formula O3 is a molecule made up of three oxygen atoms, it is an oxidant even more powerful than dioxygen. It is therefore very responsive. At ground level, it is a harmful pollutant, irritating to the lungs, but the layer it forms in the stratosphere (between 20 and 50 km above sea level) protects us from the most harmful ultraviolet rays. What is commonly called the "hole" in the ozone layer, is more precisely a thinning of this layer, accentuated by human activity. In fact, certain gases containing chlorine or bromine (widely used in refrigerators in the 1970s and 1980s), released into the atmosphere, dissociate under the effect of solar rays (UV), and release chlorine and bromine.
Oxygen-based compounds
For the same reasons as mentioned above, there are many oxygen-based compounds:
- Water of formula H2O is a molecule where oxygen is associated with two hydrogen atoms.
- Oxides are composed of oxygen bonded to other less electronegative atoms. They are formed in particular with metals (iron oxide, aluminum oxide, etc.).
- Many organic functions involve oxygen: alcohols, ketones, aldehydes, ethers, esters, carboxylic acids, carboxylic anhydrides or even amides.
Uses of oxygen
- Apart from its natural and instinctive use of living things to live, oxygen is used to understand certain phenomena, especially in paleoclimatology.
- In the modern world, oxygen has endless applications in both industrial and medical sectors.
- In medicine it is applied to assisted breathing of patients . Large reservoirs of liquid oxygen are easy to find in all hospitals. In addition, among the uses and applications of oxygen, it stands out that it is essential in the process of combustion of sugars through which most living beings obtain their energy.
- In industry, most of the oxygen (80% of world production) goes to the iron and steel industry (Bessemer process). Each ton of steel needs 3/4 ton of oxygen to obtain it.
- Similarly, when we ask ourselves what oxygen is used for, we can answer that in welding at very high temperatures . The technique is indispensable in the motor industry, in the manufacture of machinery and in aerospace, where it is used as a propellant. In addition, it also has its contribution in the manufacture of high purity metals.
Oxygen and aging
Dioxygen is the source of life for the majority of living organisms on earth, however it is also responsible for our aging. It is to them that the formation of skin wrinkles and certain cancers are attributed.
Indeed, oxygen has the property of forming free radicals, very reactive elements that can disrupt biological processes.
Chemically, a free radical is an atom (or molecule) that has lost or gained an electron. This entity is by definition very unstable because electrons always "want" to pair (which is why atoms form molecules).
The instability of free radicals gives them great reactivity with living molecules. They also tend to form new free radicals by electron exchange, which leads to many chain reactions, disrupting the normal biochemical cycle of cells. This phenomenon is also called oxidative stress.
Thus, many cosmetic brands praise the merits of antioxidant products that are effective in treating the appearance of wrinkles.
These molecules have the property of being more easily attacked than the skin, and therefore preferentially exchange electrons with free radicals to form stable elements reducing the risk of chain reaction.
However, do not dream, there is no miracle! Especially since often the active ingredients in cosmetic products are not very concentrated, which limits their field of action.
Effects of oxygen on our health
Every human being needs oxygen to breathe, but as with many substances, too much of it is not good . If one is exposed to large amounts of this element for a long time, such as breathing 50-100% at normal pressure for a prolonged period can cause damage to the lungs.
People who suffer frequent or potentially high exposures to pure oxygen at work should have a lung function check before and after performing that job . This item is normally stored at very low temperatures and therefore special clothing must be worn to prevent freezing of body tissues .
Know about more periodic elements- Aluminium, Gadolinium, Germanium, Neon, Oxygen, Potassium, Promethium, Selenium, Sodium, Terbium, Tellurium, Yttrium, Ytterbium, Zirconium
Related Articales
Recently Posted
-
भगवान गौतम बुद्ध जीवन परिचय | Gautam Buddha in Hindi
December 15, 2022. -
कार्बन के अपररूप Allotropes of Carbon in Hindi
November 5, 2022. -
मिश्र धातु किसे कहते हैं? उपयोग, नाम, गुण Alloy in Hindi
July 27, 2022. -
गलनांक किसे कहते हैं? परिभाषा, उदाहरण Melting Point in Hindi
July 20, 2022. -
परिमाप किसे कहते हैं? Perimeter in Hindi
July 19, 2022.




