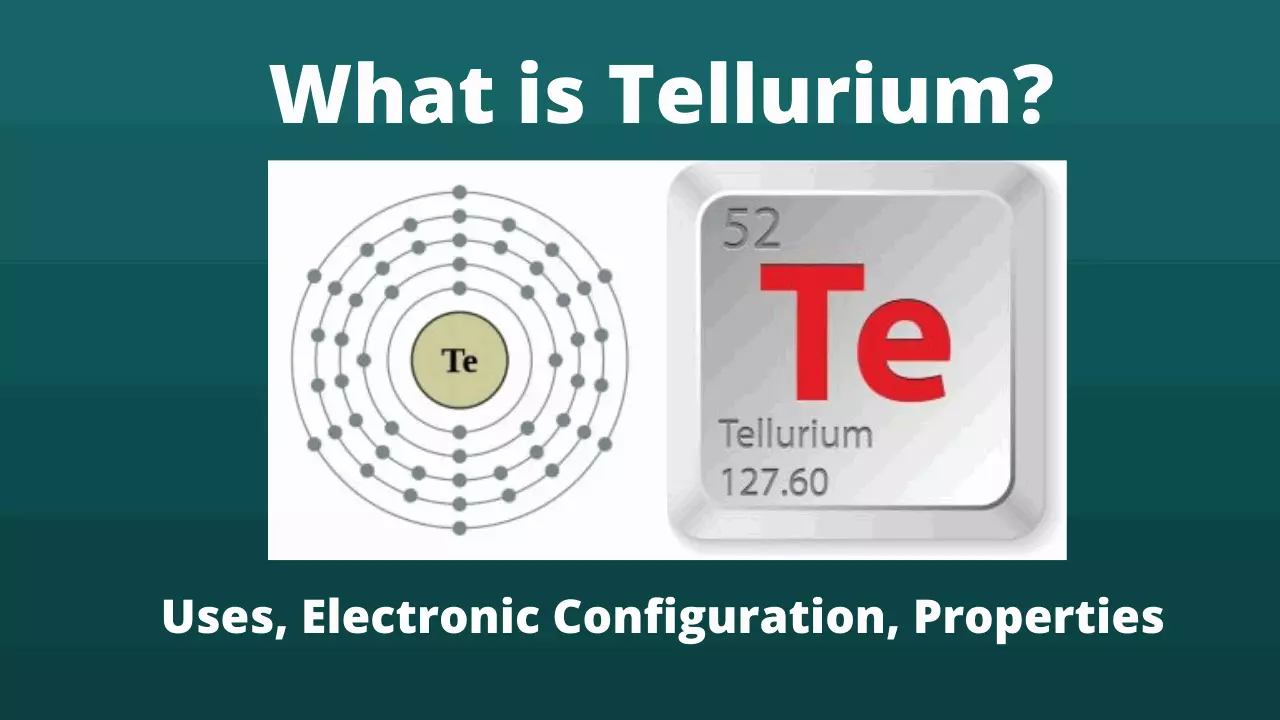
What is Tellurium? Electron configuration, Atomic Number
What is Tellurium?
Tellurium is a chemical element with atomic number 52 in the periodic table and an atomic mass of 127.60; denoted by the symbol Te (Tellurium), belongs to the family of metalloids.
Little history of Tellurium
The name of the element 52, tellurium, derived from the Latin Tellus, which corresponds to the name of the goddess named Earth. It was its discoverer, the scientist Martin Heinrich Klaproth who named it in 1789.
This name was chosen because, since its period is identical to that of selenium, whose name derived from the Greek name of the Moon, we all know that these two stars are inseparable. They are found together in the Universe just as selenium and tellurium are found together in nature.
General informations of Tellurium |
|
|
Tellurium Symbol |
Te |
|
Atomic number of Tellurium |
52 |
|
Family |
Metalloid |
|
Group |
16 |
|
Period |
5 |
|
Block |
p |
|
Volumic mass |
6.23 g.cm-3 |
|
Hardness |
2.25 |
|
Color |
Silver gray |
Atomic properties of Tellurium |
|
|
Tellurium Atomic mass |
127.60 u |
|
Atomic radius of Tellurium |
140 pm |
|
Electronic configuration of Tellurium |
[Kr] 5s2 4d10 5p4 |
|
Electrons by energy level |
2 | 8 | 18 | 18 | 6 |
|
Oxide |
Weak acid |
|
Crystal system |
Hexagonal |
Tellurium Physical properties |
|
|
Ordinary state |
Solid diamagnetic |
|
Melting point of Tellurium |
449.51 ° C |
|
Boiling point |
988 ° C |
Discovery of Tellurium
In 1782, the Austrian mineralogist Franz-Joseph Müller von Reichenstein suspected and hinted at the presence of an unknown element in gold ores in Transylvania.
More particularly in sylvanite, while he was in search of antimony present in this ore. But, since he did not find any, he concluded that there was still an unknown metal.
But it was Pál Kitaibel who encouraged research and, as explained previously, Martin Heinrich Klaproth isolated the element and named tellurium in 1798. He first proposed the Latin name tellurium for this element.
This name was chosen in reference to the terrestrial globe but also to the Roman goddess of the Earth from ancient Roman mythology.
Presence in its natural state
Tellurium is a relatively rare element since it has a clarity of 0.002 g/t. Although it is naturally a simple body, it is often present as compounds with chalcophile elements like gold or silver.
In nature, there are only a few minerals specific to tellurium such as tellurite, formerly called tellurine, with the formula TeO2 , is a very rare mineral.
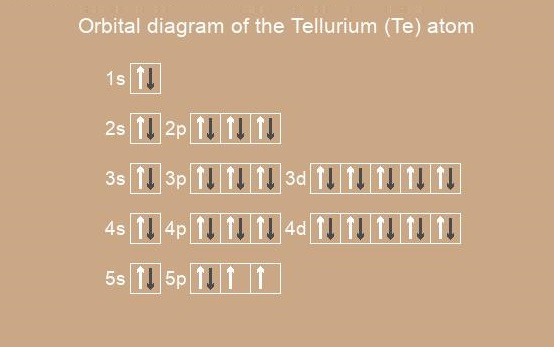
Electronic configuration of Tellurium
The Tellurium electron configuration is
Te: 1s2 2s2 2p 3s2 3p 4s2 3d10 4p 5s2 4d10 5p4
Also it can be written in Short notation (abbreviated form):
Te: [Kr] 5s2 4d10 5p4
The tellurium atom and I+1 , Xe+2 have the same electronic configuration.
Below is the electronic diagram of the Tellurium atom:
Tellurium oxidation state
Tellurium atoms in compounds have oxidation states of 6, 5, 4, 2, 1, -2.
Tellurium Ions
Below are Ions of Tellurium element:
Te6+ Te5+ Te4+ Te2+ Te1+ Te2-
Tellurium valence electrons
The tellurium valence characterizes the ability of the Te atom to form chemical bonds. Tellurium atoms in compounds exhibit valencies VI, V, IV, II, I.
Physical and chemical properties of Tellurium (Te)
Physical properties of Tellurium (Te)
- Tellurium is a crystalline metalloid solid with a silvery color.
- It is very brittle and can be very easily pulverized, resulting in a powder which can vary in color from gray to brown.
- It should be noted that tellurium is not oxidized by dioxygen at room temperature but burns with a blue-green flame, forming tellurium oxide.
- It can react with acids and bases but not with water.
- It easily combines with gold to form gold telluride.
Chemical properties of Tellurium
1- Tellurium ions in aqueous solution
Tellurium IV ion, of formula Te4+ , is a monatomic cation which has a defect of four electrons.
2- Tellurium-based compounds
- Bismuth tellurium, of formula Bi2Te3 , is a black semiconductor compound.
- Tellurium oxide, of formula TeO2 , can be produced during the combustion of pure tellurium in oxygen.
- Gold telluride, of the formula Au2Te3 , which is also called Calaverite, is in fact the most common of the compounds of gold.
3- Isotopes of Tellurium
Tellurium is a chemical element that has 38 isotopes with an atomic mass number between 105 and 142. But, in addition to its 38 isotopes, tellurium also has 17 nuclear isomers.
Among these 17 nuclear isomers, only 6 are stable:
- Tellurium 120
- Tellurium 122
- Tellurium 123
- Tellurium 124
- Tellurium 125
- Tellurium 126
And 2 have a very long period:
- Tellurium 128
- And tellurium 130
We can also consider that these 8 isotopes make up all of the natural tellurium on Earth, of which tellurium 126 is the most common with a representation equal to 18.8% of the tellurium present on Earth.
Uses of Tellurium element
Tellurium is used in different fields, below are some of the uses of Tellurium:
- Tellurium can be used as a catalyst in the vulcanization of rubber.
- Coupled with lead, it can also be used to make approximate temperature measurements.
- Tellurium is often used, associated with selenium, in laser printers and photocopiers.
- In addition, many dyes can contain tellurium.
Tellurium toxicity
Tellurium is relatively toxic to humans and causes unpleasant side effects for everyone. When tellurium is inhaled, it causes drowsiness, a feeling of dry mouth, headaches accompanied by nausea but above all a terrible body odor of garlic.
Following short-term exposure, tellurium may cause irritation to the eyes but also to the respiratory system. The liver and the central nervous system can also be affected by this chemical.
If ingested, tellurium causes garlic halo, abdominal pain, vomiting, and constipation.
Ecotoxicity of Tellurium
Tellurium, as such, does not represent a real danger to the environment since it is not harmful or easily rendered harmless by conventional processes.
However, care must be taken when tellurium is heated since tellurium chloride can emit toxic fumes of tellurium but also chlorine.
Know about more periodic elements- Aluminium, Gadolinium, Germanium, Neon, Oxygen, Potassium, Promethium, Selenium, Sodium, Terbium, Tellurium, Yttrium, Ytterbium, Zirconium
Related Articales
Recently Posted
-
भगवान गौतम बुद्ध जीवन परिचय | Gautam Buddha in Hindi
December 15, 2022. -
कार्बन के अपररूप Allotropes of Carbon in Hindi
November 5, 2022. -
मिश्र धातु किसे कहते हैं? उपयोग, नाम, गुण Alloy in Hindi
July 27, 2022. -
गलनांक किसे कहते हैं? परिभाषा, उदाहरण Melting Point in Hindi
July 20, 2022. -
परिमाप किसे कहते हैं? Perimeter in Hindi
July 19, 2022.




