What is Terbium? Electron configuration, Properties & Uses
What is Terbium?
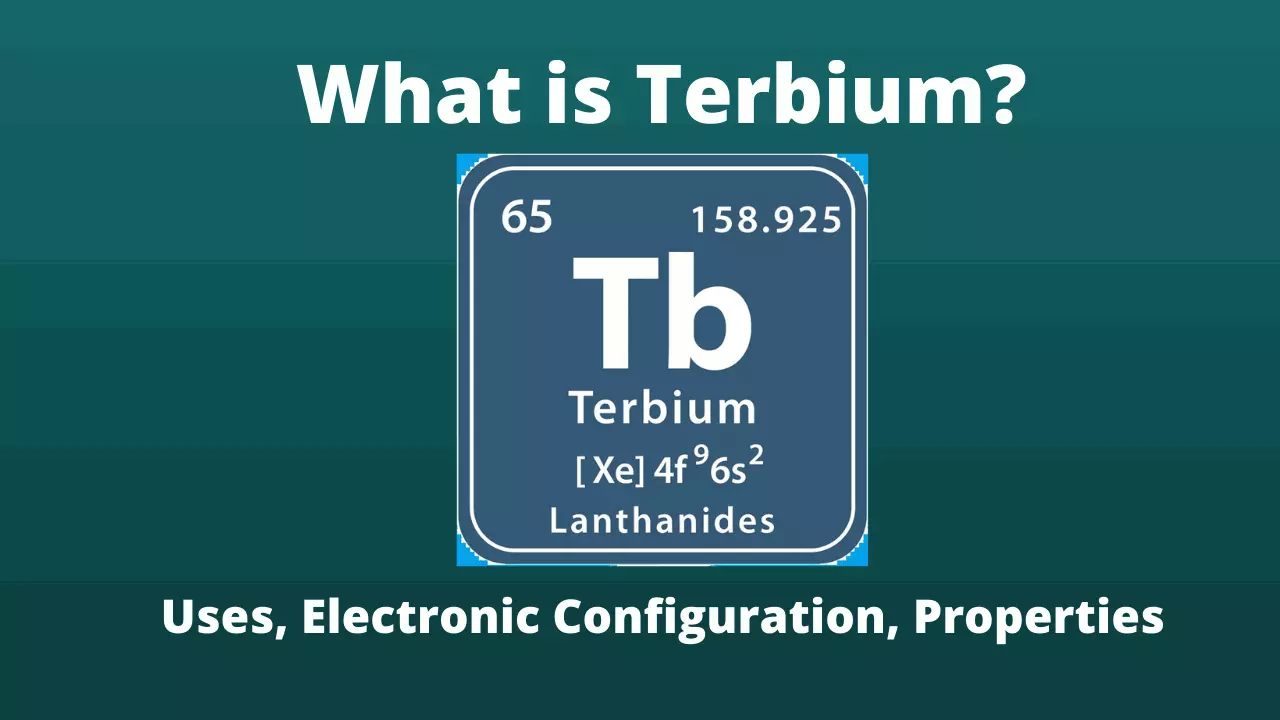
Terbium is a chemical element with atomic number 65 in the Periodic Table of the Elements. Terbium shares the same etymology as other lanthanides like erbium, yttrium and ytterbium: they come from the place Ytterby, in Sweden, where the ore from which they are extracted was discovered.
Element 65, terbium, like other rare earths, owes its name to the small Swedish village of Ytterby, whose minerals enabled the discovery of this Terbium.
General informations of Terbium |
|
|
Terbium Symbol |
Tb |
|
Atomic number of Terbium |
65 |
|
Family |
Lanthanide |
|
Period |
6 |
|
Block |
f |
|
Volumic mass |
8,230 g.cm-3 |
|
Color |
Silvery white |
Atomic properties of Terbium |
|
|
Atomic mass of Terbium |
158.92534 u |
|
Atomic radius |
175 pm |
|
Electronic configuration of Terbium |
[Xe] 6s2 4f9 |
|
Electrons by energy level |
2 | 8 | 18 | 27 | 8 | 2 |
|
Oxide |
Based |
|
Crystal system |
Compact hexagonal |
Physical properties of Terbium |
|
|
Ordinary state |
Solid |
|
Terbium Melting point |
1,356 ° C |
|
Boiling point |
3230 ° C |
Discovery of Terbium
In 1843 Terbium was discovered by the Swedish chemist Carl Gustav Mosander, who discovered it as an impurity in yttrium. Using ammonium hydroxide, he precipitated fractions of different basicity from yttrium oxide.
In these fractions, he found that the fraction that was practically colorless in solution but gave an oxide with a brown tint was terbium.
Presence in its natural state
Terbium is present in the earth's crust in association with other rare earths in many minerals such as:
- Euxenite which is composed of 1.3% terbium oxide
- Xenotime which is composed of 1% terbium oxide
- But also cerite, monazite and gadolinite
Terbium is not, contrary to its name, rare since with a Clarke of 1.2 mg / kg, it is twice as common as silver. The main deposits and mines are located in China, the United States and Brazil.
Physical and chemical properties of Terbium
Physical properties of Terbium
- Terbium is a gray colored metal that is ductile, malleable, and soft enough to be cut with a knife.
- Terbium is a stable element in air but which has two allotropic forms whose phase change occurs at 1,289 ° C.
- In the presence of oxygen, element 65 is covered with a layer of terbium oxide which can also be produced by combustion of metallic terbium.
- It can also react with water, acids and bases.
Chemical properties of Terbium
1- Isotopes of Terbium
In its natural state, terbium is a mononucleosis and monoisotopic element. Indeed, its only stable isotope is terbium 159. However, 36 synthetic radioisotopes have been characterized and their mass number is between 135 and 171.
Among these radioisotopes, the most stable are:
- Terbium 158 which has a half-life of 180 years
- And terbium 157 which has a half-life of 71 years
In addition, care must be taken as these radioisotopes decay via beta minus decay producing dysprosium atoms.
In addition to these synthetic radioisotopes, element 65 also has 27 nuclear isomers, the most stable of which is terbium 156m. This has a half-life of 24.4 hours.
2- Terbium ions in aqueous solution
The terbium III ion, of formula Tb3+ , is a monatomic ion of light pink color which has a defect of three electrons.
Uses of Terbium
Below are some of the uses of Terbium:
- Terbium is frequently used to make x-ray screens.
- Terbium is used in alloys and in the manufacture of electronic devices.
- It is also used as a stabilizer of zirconium dioxide crystals in fuel cells or as a green phosphorescent substance in cathode ray tubes.
- The bright fluorescence allows terbium to be used as a probe in biochemistry.
- It is also used as an additive for materials in solid state devices and optical fibers.
- Terbium oxide is found in fluorescent lamps and television tubes.
Toxicity of Terbium
It is important to handle terbium with care as it can be dangerous. This is because terbium vapors and gases can cause pulmonary embolism. This risk is all the more increased if the exposure becomes prolonged.
In general, terbium, like other rare earths with comparable properties, accumulates in the liver when absorbed.
Know about more periodic elements- Aluminium, Gadolinium, Germanium, Neon, Oxygen, Potassium, Promethium, Selenium, Sodium, Terbium, Tellurium, Yttrium, Ytterbium, Zirconium
Related Articales
Recently Posted
-
भगवान गौतम बुद्ध जीवन परिचय | Gautam Buddha in Hindi
December 15, 2022. -
कार्बन के अपररूप Allotropes of Carbon in Hindi
November 5, 2022. -
मिश्र धातु किसे कहते हैं? उपयोग, नाम, गुण Alloy in Hindi
July 27, 2022. -
गलनांक किसे कहते हैं? परिभाषा, उदाहरण Melting Point in Hindi
July 20, 2022. -
परिमाप किसे कहते हैं? Perimeter in Hindi
July 19, 2022.




