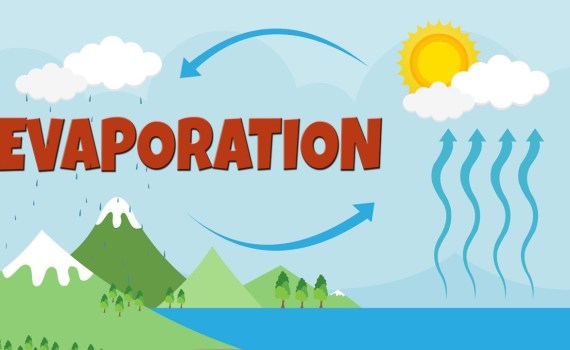
What is Evaporation?
The evaporation is a physical process which consists in a slow and progressive transition from a liquid state to a gaseous state, having acquired enough energy to overcome the surface tension.
Evaporation: what is this process
The process of transition from a liquid to a gaseous state is called vaporization. This process have two parts: evaporation and boiling .
For example, we make hot tea for ourselves. We will definitely see steam above the cup, since the water has just taken part in the boiling process.
Wait, we just said that boiling and evaporation are two different things. This is indeed the case, while these two processes can occur in parallel also.
- Evaporation is the transformation or transition of a liquid into a gas (vapor) from the free surface of the liquid. If the surface of a liquid is open and the transition of a substance from a liquid to a gaseous state begins from it, this will be called evaporation.
- Boiling is a process of intense vaporization that occurs in a liquid at a certain temperature.
Remember evaporation can occur without boiling, just then it will not be noticeable to us. For example, the water in the lake evaporates, although we do not notice it. Boiling is essentially an intense evaporation caused by external conditions – bringing a substance to the boiling point.
Physics explains evaporation by the fact that the liquid is usually slightly colder than the surrounding air – due to this temperature difference, evaporation occurs.
If there are no external influences, the evaporation of liquids occurs extremely slowly. The molecules leave the liquid due to the diffusion phenomenon .
It is interesting that the direction of heat flux during evaporation can go in different sequences and combinations:
- from the depth of the liquid to the surface, and then into the air;
- only from liquid to surface;
- to the surface of water and gaseous environment at the same time;
- to the surface area only from the air.
What is the main difference between evaporation and boiling:
| Evaporation | Boiling |
| At any temperature, from the surface of the liquid | At a certain temperature, in the entire volume of liquid |
Evaporation at the molecular level
Let’s remember the features of different states of aggregation of matter.
| Aggregate states | Properties | Molecule arrangement | Distance between molecules | Movement of a molecule |
| Solid | retains its shape and volume | in the crystal lattice | correlates with the size of the molecules | fluctuates around its position in the crystal lattice |
| Liquid | keeps volume | chaotic | close to each other | are inactive, when heated, the speed of movement of molecules increases |
| Gases | occupy the provided volume | chaotic | more molecular sizes | chaotic and continuous |
It can be seen from this table that the molecules in liquids are close to each other, but chaotically, that is, they do not have a crystal lattice, as in solids. These molecules move (moreover, the higher the temperature, the faster they move) and collide during the movement. Collisions change the direction and speed of movement – because of this, molecules sometimes quickly rush to the surface of the liquid and fly out of it. This is evaporation.
In the previous paragraph, it was not coincidence that we noticed that molecules move faster with increasing temperature – because of this, evaporation is more intense. In this case, cooling occurs: all the fastest molecules have already left the heated liquid and the temperature of the liquid itself decreases.
What is Evaporation rate?
The amount of liquid that evaporates from a unit of surface area per unit of time.
OR
Evaporation rate is the amount of water that evaporates from a surface of 1 cm2 in one second.
OR
Evaporation rate is the amount of liquid that evaporates from the free surface per unit of time.
The rate of evaporation depends on the following factors:
- Surface temperature– The higher the temperature, the more evaporation. After the rain in Himalayan regions, the streets remain wet for a long time, but in Jaipur, even in the rainy season, everything dries up quickly due to the high temperature.
- Wind– The higher the wind speed, the more evaporation. A hair dryer works on this principle – in fact, it creates a portable wind that helps dry your hair.
- Moisture deficiency– The evaporation rate will be higher where there is a greater moisture deficit. It is unlikely that many of us have been to the Sahara, but what it is all represent. In any desert, humidity is colossally low – because of this, evaporation is more intense.
- Pressure– The higher the pressure, the less evaporation. We have already found out that despite the difference between boiling and evaporation, the two processes are related. Thus, the boiling point of water at the summit of Mount Everest is 69 degrees Celsius. While in our daily life it is equal to 100. This brings us back to the first factor – temperature.
Evaporation in life
And really: what does not evaporate in this life – we meet with this every day. Let’s find out why this process is needed at all, and how people have learned to benefit from it.
Evaporation in humans and animals
Above, we discussed the question of why if we pour ourselves over with warm water, we will still get cold. The feeling of coldness after sweating works by the same principle – at some point we feel cold.
Sweating itself is an important process of thermoregulation of the body. If we reach a high temperature (due to external influences or due to illness), then the body tends to cool itself so as not to die due to the transformation of proteins in our body into scrambled eggs.
Sweat is released through the pores of the skin, and then evaporates – all this allows our body to quickly get rid of excess energy, cool the body and normalize the temperature.
At high humidity, cold and heat are perceived more sensitively. This is due to the person sweating at high temperatures. This mechanism helps us fight heat and “throw off” excess heat, but with high humidity, sweat cannot evaporate.
In low humidity , something similar happens. Oddly enough, in cold weather we also sweat (much less, but still it happens). If the humidity outside is low, sweat will evaporate from under the jacket and we will be comfortable. And with high humidity, it will linger there and will conduct heat outside, taking precious Joules of heat from us.
In animals, this mechanism works in a similar way. But, for example, evaporation from the skin is not enough for dogs, so they often open their mouths, stick out their tongue and breathe sometimes well, very funny.
It is the throat and tongue of the dog that are ideal for evaporating moisture and cooling the animal’s body.
Evaporation in plants
Surprisingly, the evaporation mechanism in plants also works in a similar way. Plants love water very much, so we water our houseplants, but in the deserts they simply do not exist.
They can evaporate the water that the flowers have absorbed so as not to overheat under the hot sun. Yes, water is needed for plants to feed, but on hot days it is also needed for temperature self-regulation. Therefore, do not forget to water the flowers, and on very hot days, do it even more intensely.
Evaporation in nature and the environment
The evaporation process is directly related to the water cycle in nature. It is the water cycle in nature that ensures life on Earth – since moisture is spread throughout the world, plants in the wild are able to live without our attempts to water a large palm tree from a watering can.
Evaporation of water from the surface of rivers, lakes, seas and oceans creates rain clouds, which then, pouring rain, water plants and trees. Many people do not like rain, they say, it is wet, disgusting and flows into their shoes, but it is very much needed in arid regions, which often suffer from drought.
Evaporation in industry and everyday life
With everyday life, everything is quite simple: we dry things, prepare food, buy air humidifiers or smear a spilled puddle on the floor.
In the case of industry, things are not so obvious to us. Evaporation-based industrial technology is designed in a similar way: it always maximizes the surface area of the liquid so that evaporation is intense.
For example, the evaporator shown in the diagram consists of a set of interconnected evaporators. Its action is based on steam produced in one stage, which is used as a heat source for the next stage. As the temperature decreases from one stage to the next, the vacuum increases so that the boiling point becomes lower and evaporation is maintained. It is designed to remove waste from water.
Industrial and chemical uses of Evaporation:
Considered as a unitary operation, evaporation is used to remove the vapor formed by boiling a liquid solution or suspension to obtain a concentrated solution. This can be done by heating or at reduced pressure. In the vast majority of cases, evaporation seen as a unitary operation refers to the removal of water from an aqueous solution.
Vacuum evaporation is used in the food industry for food preservation and in other industries for coating various materials.
Evaporative coolers (evaporative cooling), which can significantly cool a building by simply blowing dry air over a water-saturated filter.
The use of evaporation on dry or concentrated samples is a common preparatory step for many laboratory analyzes such as spectroscopy and chromatography . Systems used for this purpose include rotary evaporators and centrifugal evaporators .
When clothes are hung on a clothesline, even if the ambient temperature is below the boiling point of water, the water will evaporate. This is accelerated by factors such as low humidity, heat (from the sun) and wind. In a dryer, hot air is blown into the clothes, allowing the water to evaporate very quickly.
Evaporation of water from a brine produces flame salt .
what is evaporation in the water cycle?
Evaporation: from liquid to gas or vapor
The reason for evaporation
Evaporation is the process by which liquid water turns into gas or vapor. In the water cycle, evaporation is the primary way in which liquid water turns into water vapor in the atmosphere. Oceans, seas, lakes and rivers provide approximately 90% of the moisture in our atmosphere through evaporation. The remaining 10% from plant transpiration.
Heat (energy), supplied by the sun, is necessary for evaporation. Energy is used to break down water molecules, causing rapid evaporation at the boiling point (100 ° C) and slower evaporation at the freezing point. When the relative humidity of the air is 100% (a state of saturation), evaporation cannot take place. Evaporation decreases the heat of the environment, which is why the water that evaporates from your body cools you down.
Evaporation and the water cycle
Evaporation from the oceans is the primary way for water to enter the atmosphere. The large surfaces of the oceans (more than 70% of the Earth is covered by the oceans) allow large-scale evaporation. On a global scale, the amount of water that evaporates is almost the same as the amount of water that falls back. Although it varies geographically. Evaporation is more common over oceans than precipitation, while over land, precipitation is more frequent than evaporation. Most of the water evaporated from the oceans ends up in the oceans as precipitation. Only 10% of the water evaporated from the oceans is carried over land and falls back as precipitation. Once evaporated, a water molecule spends about 10 days in the air.
Related Articales
Recently Posted
-
भगवान गौतम बुद्ध जीवन परिचय | Gautam Buddha in Hindi
December 15, 2022. -
कार्बन के अपररूप Allotropes of Carbon in Hindi
November 5, 2022. -
मिश्र धातु किसे कहते हैं? उपयोग, नाम, गुण Alloy in Hindi
July 27, 2022. -
गलनांक किसे कहते हैं? परिभाषा, उदाहरण Melting Point in Hindi
July 20, 2022. -
परिमाप किसे कहते हैं? Perimeter in Hindi
July 19, 2022.




