Hydrogen- Electron Configuration, Properties, Valency, Uses
What is Hydrogen?
Hydrogen is a chemical element that is numbered 1 in the Periodic Table of the Elements. Hydrogen symbol is H. The hydrogen atom has the simplest structure: the nucleus consists of one proton and one electron. Hydrogen is part of the main substance of the Earth, i.e. water.
Hydrogen belongs to fairly common elements, occurs naturally in a free state and in the form of compounds ( water, oil, organic matter). It is rarely found in free form. Hydrogen is the most abundant element in space.
Under normal conditions, hydrogen is a colorless and odorless gas, almost 15 times lighter than air. Hydrogen has a very high thermal conductivity, comparable to the thermal conductivity of metals.
The word hydrogen is formed by the prefix -hydro, coming from the ancient Greek hydor, meaning water, and the suffix -gene , coming from the ancient Greek gennao, meaning who generates.
It was the French chemist Antoine Lavoisier, who proposed this name (Hydrogen) in 1783. Indeed, thanks to a hydrolysis of water, it was discovered that hydrogen and oxygen were components of water, hence the etymological meaning of "water former".
Hydrogen becomes highly flammable when it is in its monatomic gaseous form, where it reacts spontaneously with certain oxidizing elements such as chlorine, oxygen and fluorine.
This, while it is in its pure state, cannot dissolve in water, but it does perform this action on many metals, which is why it is ideal in the metallurgy process. It is highly soluble in amorphous and crystalline metals, in rare earths and in transition metals.
General Properties of Hydrogen |
|
|
Hydrogen Symbol |
H |
|
Atomic number |
1 |
|
Family |
Non-metal |
|
Group |
1 |
|
Period |
1 |
|
Block |
s |
|
Volumic mass |
0.08988 gL -1 |
Atomic properties of Hydrogen |
|
|
Hydrogen Atomic mass |
1.00794 u |
|
Atomic radius |
25 pm |
|
Electronic configuration of Hydrogen |
1s1 |
|
Electrons by energy level |
1 |
|
Crystal system |
Hexagonal |
Electronic Configuration of Hydrogen
Hydrogen in the periodic table takes 1st place, in 1st period. This means it contains 1 electron, so electronic configuration of Hydrogen will be- 1s1 .
It has a melting point of -259.34 degrees C. and a boiling point of -252.87 degrees C.
Important questions regarding Hydrogen-
What is the valency of hydrogen?
Hydrogen atoms in compounds exhibit valency I.
What are the oxidation states of hydrogen?
Hydrogen atoms in compounds have oxidation states 1, 0, -1.
What are the quantum numbers of hydrogen?
Quantum numbers are determined by the last electron in the configuration; for the H atom, these numbers have the value N = 1, L = 0, M l = 0, M s = ½
What is the Ionization energy of the hydrogen element?
The energy spent on the separation of an electron from an atom is called the ionization energy and is denoted by Eo. The Ionization energy of H is- Eo = 1312 kJ/mol
Discovery of Hydrogen
The release of combustible gas during the interaction of metals and acids was observed as early as the 16th century, that is, during the formation of chemistry as a science.
In 1671 the Irish chemist Robert Boyle studied the effects of dilute sulfuric acid on iron and observed that there is production of a flammable gas which he did not identify as a new compound but which actually corresponds to dihydrogen.
The famous English scientist Henry Cavendish studied the substance in 1766 and gave it the name "combustible air.
When burned, this gas produced water. Unfortunately, the scientist's adherence to the theory of phlogiston (hypothetical "superfine matter") prevented him from coming to the correct conclusions.
It shows that it can be obtained by reaction between various acids and metals such as iron, zinc or tin and highlights characteristics that distinguish it from known gasses: it is less dense than air and can burn explosively.
The French chemist and naturalist Antoine Lavoisier, with the help of special gas meters, in 1783 carried out the synthesis of water, and then its analysis by means of decomposition of water vapor with hot iron. Thus, scientists were able to come to the correct conclusions. They found that "combustible air" is not only a part of water, but can also be obtained from it.
In 1787, Lavoisier put forward the assumption that the gas under study is a simple substance and, accordingly, belongs to the number of primary chemical elements. He named it hydrogene (from the Greek words hydor - water + gennao - who give birth), that is, "giving birth to water."
In 1800 English chemists William Nicholson and Anthony Carlisle showed that it can be obtained by electrolysis of water.
Two new isotopes of hydrogen were subsequently discovered, deuterium (in 1932) and tritium (in 1934).
Presence of Hydrogen
Hydrogen is the most abundant element in the Universe. Indeed, it represents 92% by number of atoms, 75% by mass. It is predominantly present in stars and gaseous outer planets with a solid core. It is also the main component of nebulae and interstellar gas.
However, it only represents 0.22% of atoms in the earth's crust. It is therefore far behind oxygen, which represents 47% of atoms, and silicon, which represents 27% of atoms.
It is also rare in the Earth's atmosphere. In fact, it represents, by volume, only 0.55 ppm of atmospheric gases. The most common source of hydrogen on Earth is water, the molecule of which is made up of two atoms of hydrogen for one atom of oxygen. Hydrogen is also and above all the main constituent, in atomic number, of all living matter. It is associated with carbon in all organic compounds.
For example, hydrogen represents 63% of atoms and 10% of the mass of the human body.
Hydrogen, subjected to very low pressure when in space, tends to exist as individual atoms since it does not collide with other atoms to combine. We can then find clouds of hydrogen which are at the base of the process of star formation.
Physical and chemical properties of hydrogen
Simple body of hydrogen
Hydrogen is the first element of the periodic table. Its nucleus has only one proton and its main isotope is even devoid of neutrons (a unique case) which makes the hydrogen atom the simplest, smallest and lightest of atoms.
It also occupies a somewhat special status in the periodic table: its place in the first column could have earned it belonging to the alkali family while the electron missing to complete its outer layer could have linked it to the family. halogens. However, its properties distinguish it so clearly from these two families that it has by default been classified as "non-metal".
At room temperature and atmospheric pressure, hydrogen is present as a colorless, odorless, sparse gas which burns explosively on contact with air to produce water.
It can be produced by reaction between an acid and a metal or by electrolysis of water.
Hydrogen in the form of a single body, except at extremely low pressures as in intergalactic space or extremely high as in the central parts of Jupiter and Saturn, is formed of molecules of hydrogen of the formula H2 .
When hydrogen is subjected to extremely high pressures, it is said to be in a so-called "dark" state, an intermediate state between gas and metal. In this state, it does not reflect or transmit light, but it also becomes a very weak conductor of electricity. It can then be compared to the following alkali metals, in group 1 of Mendeleev's periodic table of elements.
While at lower pressures hydrogen is present as a monatomic gas.
Hydrogen gas
Under normal conditions of pressure and temperature, that is to say conditions that are of interest to chemistry and earth sciences, hydrogen is present in the form of a molecular gas of hydrogen, of the formula H2 . In fact, dihydrogen is capable of forming molecular clouds in galaxies at the origin of star formation.
As said before, at low pressure and high temperature, hydrogen is present in the form of monoatomic gas, of formula H. It is in this form that we can find it in space, as interstellar gas or intergalactic.
It is because of the vastness of space, and despite the low density of the gas, that monoatomic hydrogen constitutes about 75% of the baryonic mass of the Universe.
Solid hydrogen
It is possible to obtain solid hydrogen by lowering the temperature below the melting point of hydrogen, that is to say to 14.01 K, or -259.14 ° C.
This state was obtained for the first time in 1899 by James Dewar.
Metallic hydrogen
When subjected to very high pressures and at very low temperatures, hydrogen reaches a so-called metallic phase. Some believe that there is a range of pressures under which, even when subjected to very low temperatures, metallic hydrogen is liquid.
Hydrogen isotopes
By losing its single electron, hydrogen will give an H + ion frequently called a proton, it is the most abundant isotope.
The proton cannot exist in solution in the free state, it is always linked to the electronic cloud of a molecule while in aqueous solution it is solvated by water molecules. It thus forms the hydronium ion H 3 O + , it is also called oxonium or hydroxonium.
The hydrogen atom can also get a second electron to get the hydride ion of formula H- . It then has the same stable electronic procession as the helium atom.
In addition, hydrogen is the only element whose different isotopes have distinct names and sometimes even their own symbol.
The major isotope is protium, so called because it has only one proton in its nucleus and the other main isotopes are deuterium (symbol D) and tritium (symbol T).
There are other isotopes (tetranium A = 4, pentium A = 5, hexium A = 6 and septum A = 7) but these are radioactive and highly unstable with half-lives all well below one second. .
Hydrogen ions
In aqueous solution, hydrogen forms the cation of formula H+ consisting of a single proton.
This ion, called hydronium ion, hydroxonium ion or oxonium (these terms are synonymous) combines with a water molecule to form the H3O+ ion .
This is the ion responsible for acidity, the latter increases and the pH decreases when the concentration of H3O+ ion increases.
In addition, these ions are present even in pure water because it is the seat of a phenomenon called autoprotolysis of water which leads the water molecules to form hydrogen cations and hydroxide ions depending on the equilibrium :
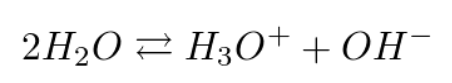
Production
In order to obtain hydrogen in the most economical way possible, hydrocarbon reforming is used. Even if in practice, the most used method is the steam reforming of natural gas mainly composed of methane.
At a vapor of between 700 and 1,100 °C, the water vapor reacts with methane to give carbon monoxide and hydrogen. The mixture of hydrogen and carbon monoxide is commonly referred to as synthesis gas.
The purification of hydrogen is easier under high pressure. This is why the reforming is carried out under a pressure of twenty atmospheres. To increase the production of hydrogen, the reaction must take place in the presence of excess water vapor. Indeed, the carbon monoxide will be oxidized to a higher oxidation level which will therefore lead to obtaining carbon dioxide.
How can hydrogen be obtained?
For it to be possible to use hydrogen in so many different ways, it is first necessary to extract it from the compounds in which it is found, be it from water, some fossil fuel or even organic matter .
Electrolysis
This process breaks down water to obtain hydrogen and oxygen. To carry out this reaction it is necessary to have an electrical supply, since the passage of electrical current causes the water to divide. In this way hydrogen is formed at the cathode and oxygen at the anode.
biomass
- Biomass is matter that comes from living beings and is abundant in hydrogenated compounds. Through chemical treatments and processes, biomass can be converted into a gas, known as biogas, and biogas in turn can be transformed into hydrogen.
- There are other processes, where liquid biofuels can be obtained from biomass that can also be used to produce more transportable Hydrogen.
Fossil fuels
Fossil fuels contain hydrogen in their composition. To obtain it in gas form, the water would have to be reacted, using a catalyst to make it react more easily. This process is known as steam reforming and, like electrolysis, an energy input will also be necessary, since it is an endothermic process in which the extraction of hydrogen and carbon monoxide would be obtained.
What uses can hydrogen have?
- Hydrogen is primarily used for the production of ammonia and many other organic compounds.
- Hydrogen is used as a reagent but also has other applications in engineering and physics. It is useful as a shielding gas applied in welding methods such as atomic hydrogen welding.
- It was also widely used as a gas to inflate airships. But that changed following the Hindenburg accident in 1937 since it will be replaced by helium.
- Hydrogen is also used for the production of hydrogen bombs, also known as H bombs, in which the hydrogen fuses together to form helium.
- Hydrogen 3, also called tritium, is generated in nuclear reactors and used to produce hydrogen bombs, as a source of radiation for luminous paints, and as an isotopic marker for life sciences.
- In current applications, mixed or pure hydrogen together with nitrogen is used as a tracer gas with which leaks are detected. This gas is known by the name of forming gas.
- Liquid hydrogen is involved in cryogenic research, especially in superconductivity studies.
- It is used to cool generators found in power plants because this element has the highest thermal conductivity of gases.
- Hydrogen is the source from which hydrochloric acid is made.
- Hydrogen is used as a hydrogenating agent, mostly when raising the saturation level of oils and unsaturated fats that are found in products such as margarine and also in the manufacture of methanol .
Uses of hydrogen as fuel
Hydrogen can function as a green fuel as it does not produce greenhouse gases. It has already been used in various space projects, as fuel for rocket propulsion. Currently, a less expensive way is being sought to extract hydrogen en masse in order to commercialize its use in automobiles.
Since 1980, countries such as Japan, Canada, the United States and some other member countries of the European Union have supported the research and development of this technology. As a consequence, today in the world there are several companies marketing fuel cells made with hydrogen as the main component.
Uses of hydrogen as a source of energy
Once the hydrogen and its uses are extracted, it becomes an energy vector and the energy it produces can be stored and delivered, generating large amounts of electricity. On the other hand, hydrogen can also be burned to run an engine that generates energy.
Regardless of the way in which the energy is generated, we can be convinced that hydrogen is an interesting proposal. Since it can be extracted from water, it would become an ecological and viable source of energy for a sustainable future.
Related Articales
Recently Posted
-
भगवान गौतम बुद्ध जीवन परिचय | Gautam Buddha in Hindi
December 15, 2022. -
कार्बन के अपररूप Allotropes of Carbon in Hindi
November 5, 2022. -
मिश्र धातु किसे कहते हैं? उपयोग, नाम, गुण Alloy in Hindi
July 27, 2022. -
गलनांक किसे कहते हैं? परिभाषा, उदाहरण Melting Point in Hindi
July 20, 2022. -
परिमाप किसे कहते हैं? Perimeter in Hindi
July 19, 2022.




